Streamline breast cancer biomarker quantification on a single platform and implement this comprehensive AI-assisted panel to boost pathology workflow efficiency

Improve Workflow Efficiency
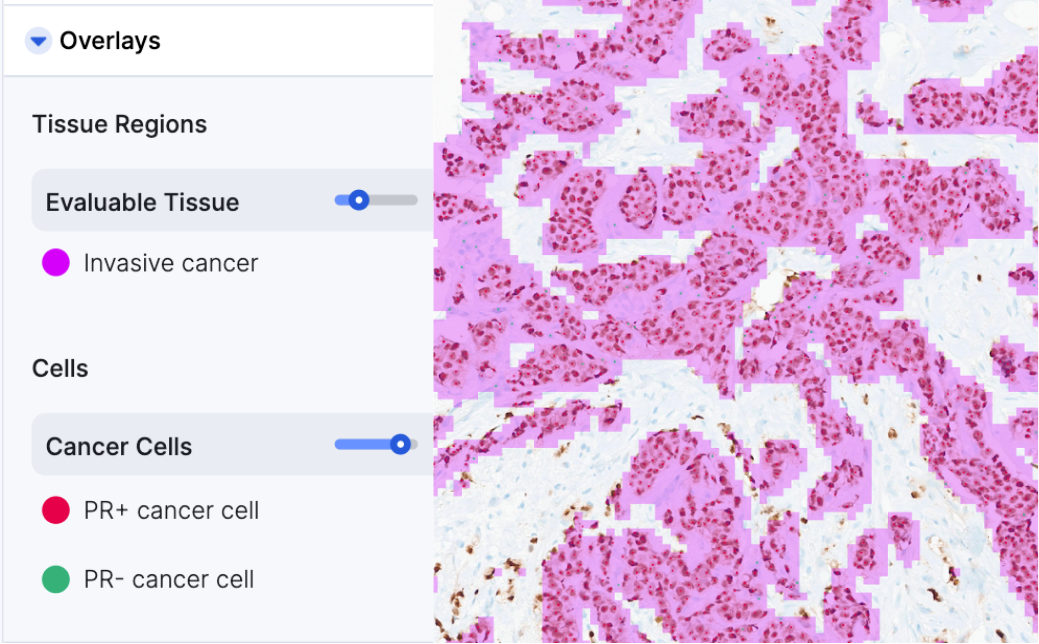
AI-generated scoring of Breast Cancer IHC is fully integrated in to the AISight® Image Management System reducing the time and effort required for manual assessments.
.png?width=100&height=100&name=Kaylee%20Workbook%20(1).png)
Full Spectrum Biomarker Analysis
The AIM-IHC Breast Panel consolidates analysis for key critical biomarkers, including HER2, ER, PR, and Ki-67 - all available on a single platform

Fast and Accurate Quantification
Achieve reliable, and accurate biomarker quantification with zero-clicks in AI-predicted areas of invasive cancer - no longer compromise accuracy for speed
-2.png?width=2000&height=911&name=Untitled%20design%20(2)-2.png)
Specifications:
- Intended Use: Research Use Only
- Indications: Breast Cancer
- Clones: Agilent HercepTest, Roche Ventana 4B5
- Scanners: Leica Aperio® AT2 and GT450, Hamamatsu NanoZoomer® s360
- Inputs: Breast cancer biopsy, resection, and/or excision sample from primary, recurrent, or metastatic tumor (excluding in situ tumor)
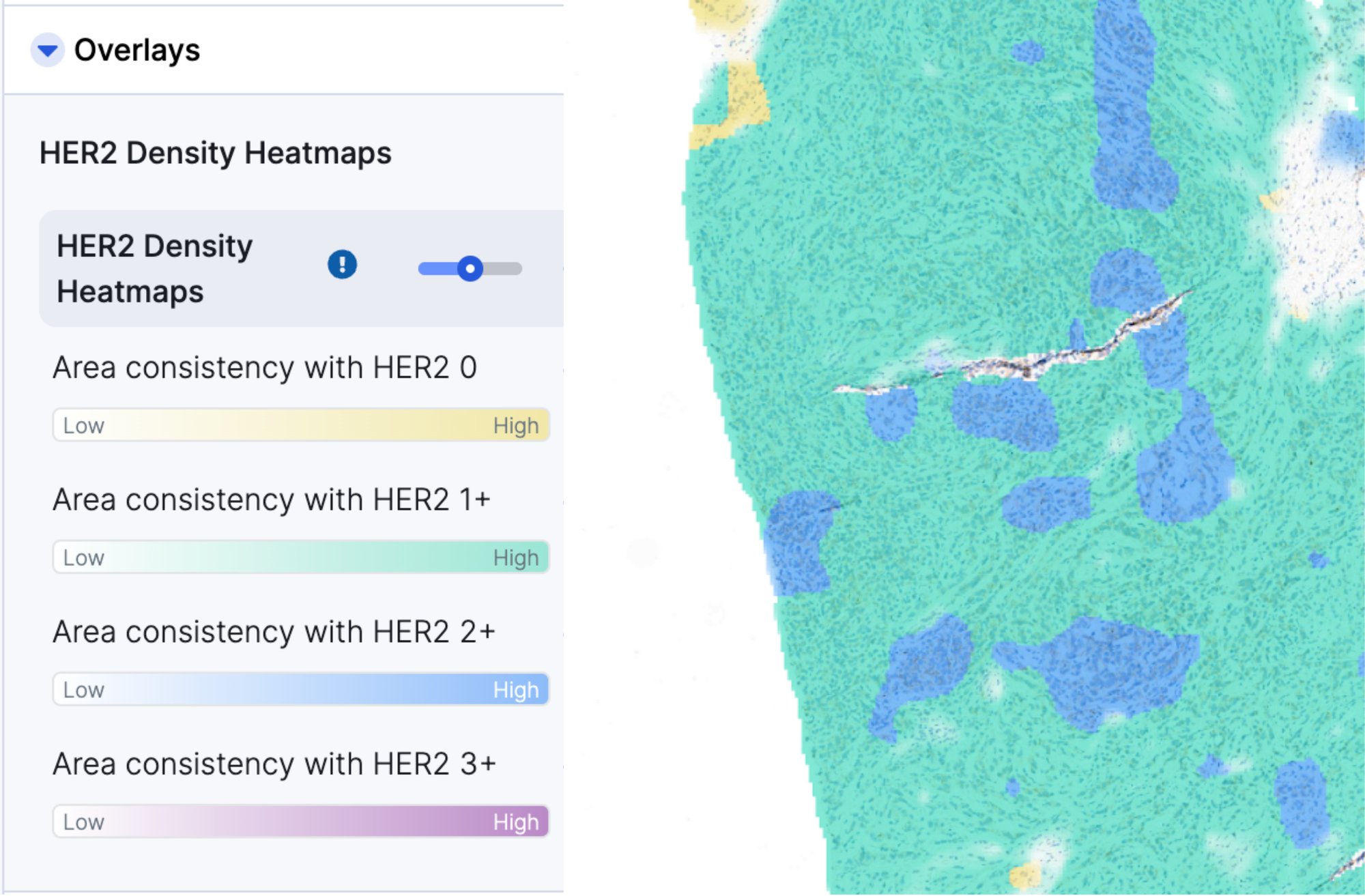
- Outputs: HER2 score (0, 1+, 2+, 3+), Area of invasive carcinoma, Additive multiple instance learning (aMIL) density heatmap
AIM-HER2 Breast may be compatible with additional scanner types. Please contract us for more information
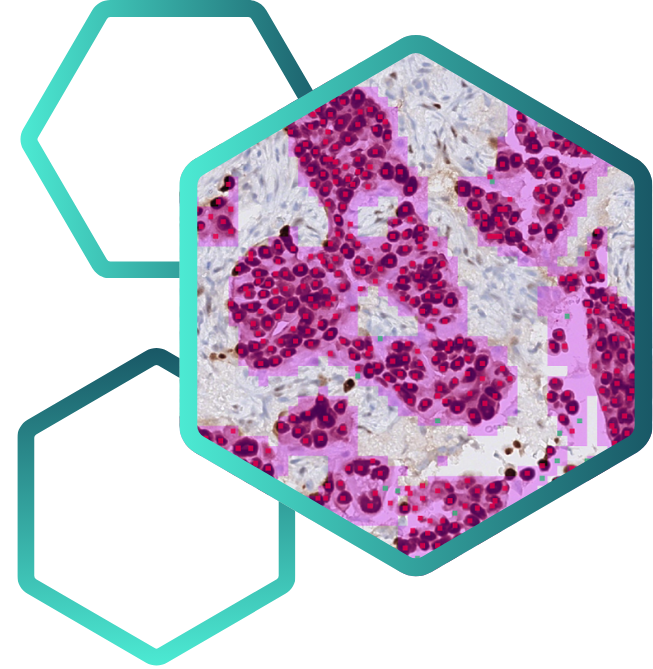
AIM-HER2 Breast
AIM-HER2 Breast is multi-clone and multi-scanner compatible
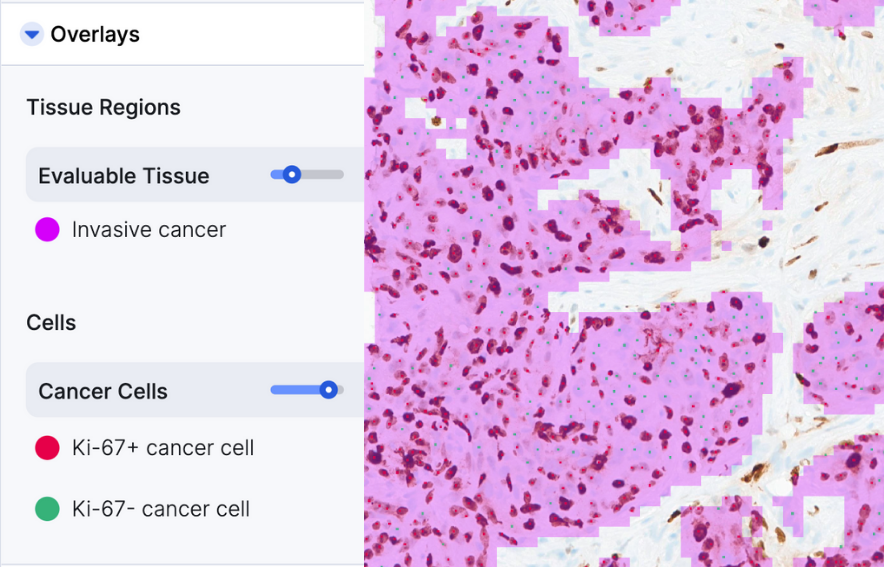
AIM-Ki67 Breast
AIM-Ki-67 Breast is multi-clone and multi-scanner compatible
Specifications:
- Intended Use: Research Use Only
- Indications: Breast Cancer
- Clones: Agilent Dako MIB-1; Roche Ventana 30-9
- Scanners: Leica Aperio® AT2 and GT450; Philips UFS, Roche DP200 and DP600, Hamamatsu NanoZoomer® s360
- Inputs: Breast cancer biopsy, resection, and/or excision sample from primary, recurrent, or metastatic tumor (excluding in situ tumor)
- Outputs: Percent Ki-67+ positive cancer cells, Area of invasive cancer, Cell counts for Ki-67+ cancer cells and total cancer cells in invasive cancer regions
Specifications:
- Intended Use: Research Use Only
- Indications: Breast Cancer
- Clones: Agilent Dako EP1; Roche Ventana SP1
- Scanners: Leica Aperio® AT2 and GT450; Philips UFS, Roche DP200 and DP600, Hamamatsu NanoZoomer® s360
- Inputs: Breast cancer biopsy, resection, and/or excision sample from primary, recurrent, or metastatic tumor (excluding in situ tumor)
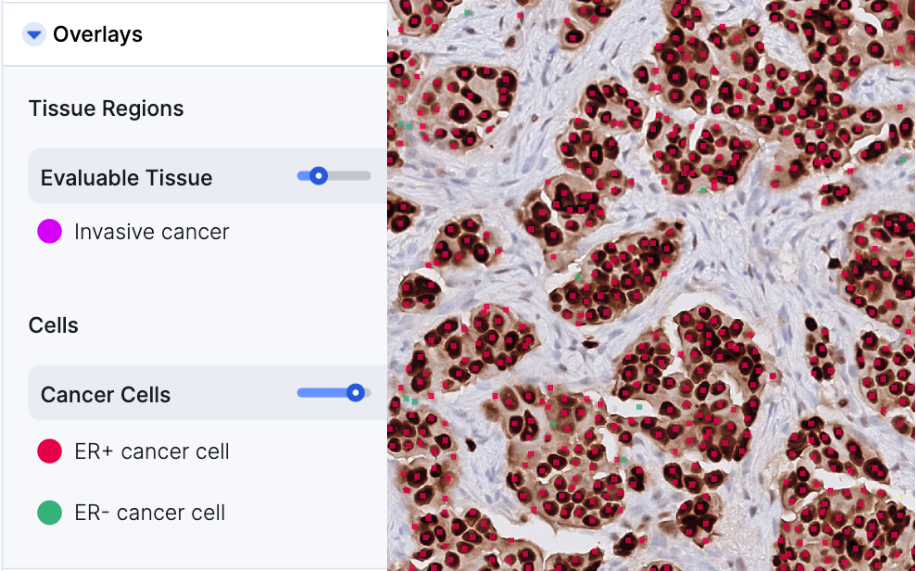
- Outputs: Percent ER+ positive cancer cells, Area of invasive cancer, Cell counts for ER+ cancer cells and total cancer cells in invasive cancer regions
AIM-ER Breast
AIM-ER Breast is multi-clone and multi-scanner compatible
AIM-PR Breast
AIM-PR Breast is multi-clone and multi-scanner compatible
Specifications:
- Intended Use: Research Use Only
- Indications: Breast Cancer
- Clones: Agilent Dako PgR 636; Roche Ventana 1E2
- Scanners: Leica Aperio® AT2 and GT450; Philips UFS, Roche DP200 and DP600, Hamamatsu NanoZoomer® s360
- Inputs: Breast cancer biopsy, resection, and/or excision sample from primary, recurrent, or metastatic tumor (excluding in situ tumor)
- Outputs: Percent PR+ positive cancer cells, Area of invasive cancer, Cell counts for PR+ cancer cells and total cancer cells in invasive cancer regions
CASE STUDY
AI-Assisted Titer Selection in Early Assay Development
- PathAI deployed IHC Explore on prostate cancer specimens stained with a novel, in-development assay
- IHC Explore quantifies staining intensity at single-cell resolution, enabling rapid assay characterization and titer optimization
- Continuous staining intensity measurement provides added value for next-generation biomarkers and precision medicine strategies
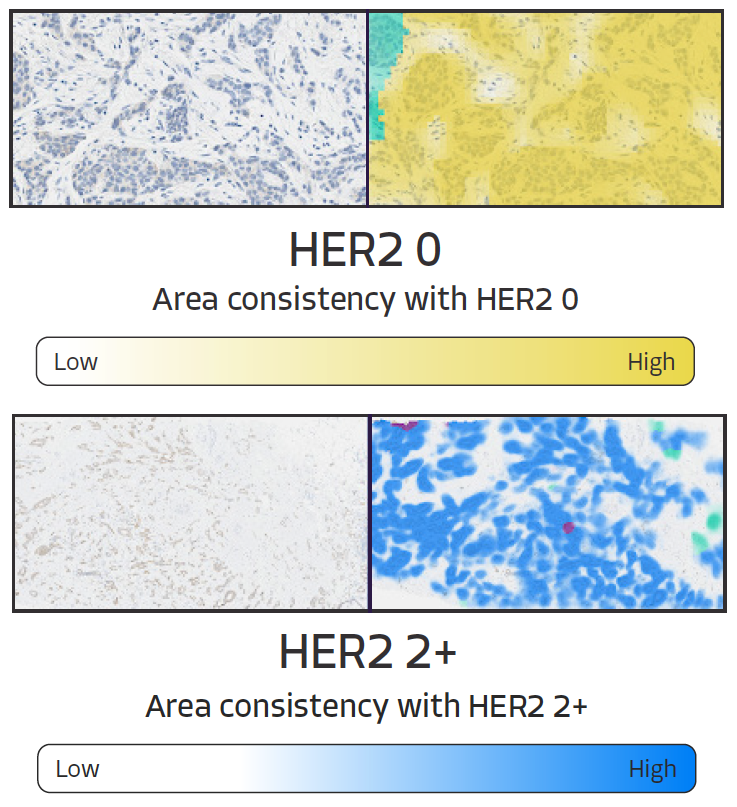
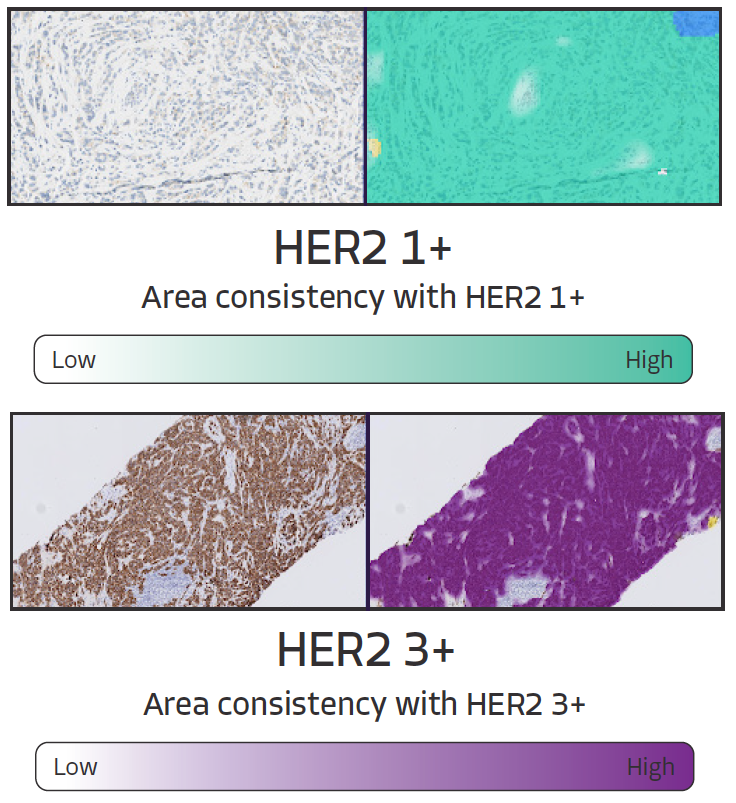
HER2 Scoring Heatmaps - Additional Information
Additive heatmaps highlight tissue patterns features that most contribute to AIM-HER2's score prediction. Pathologists are able to more confidently assess HER2 expression, especially in borderline cases with more slide-level heterogeneity as they can quickly and more easily focus on the most critical features and slide areas.
AIM-IHC Breast Panel, AIM-HER2, AIM-ER, AIM-PR, and AIM-Ki-67 are for research use only. Not for use in diagnostic procedures.
AISight is for Research Use Only in the US; AISight Dx is CE-IVD marked in Europe, UK and Switzerland.
