PathAI Digital Diagnostics
Webinar Series
AISight® Dx Digital Pathology Platform:
Live Deep Dive
.png?width=400&height=400&name=Landing%20page%20header%20use%20graphic%20(1).png)
October 23, 2025, 11:00 AM–12:00 PM ET
AISight® Dx is the first digital pathology image management system to secure FDA clearance with an authorized Predetermined Change Control Plan (PCCP), supports the broadest range of whole slide imaging scanners used across pathology laboratories.
Join us to learn how AISight Dx supports modern pathology workflows at enterprise scale.
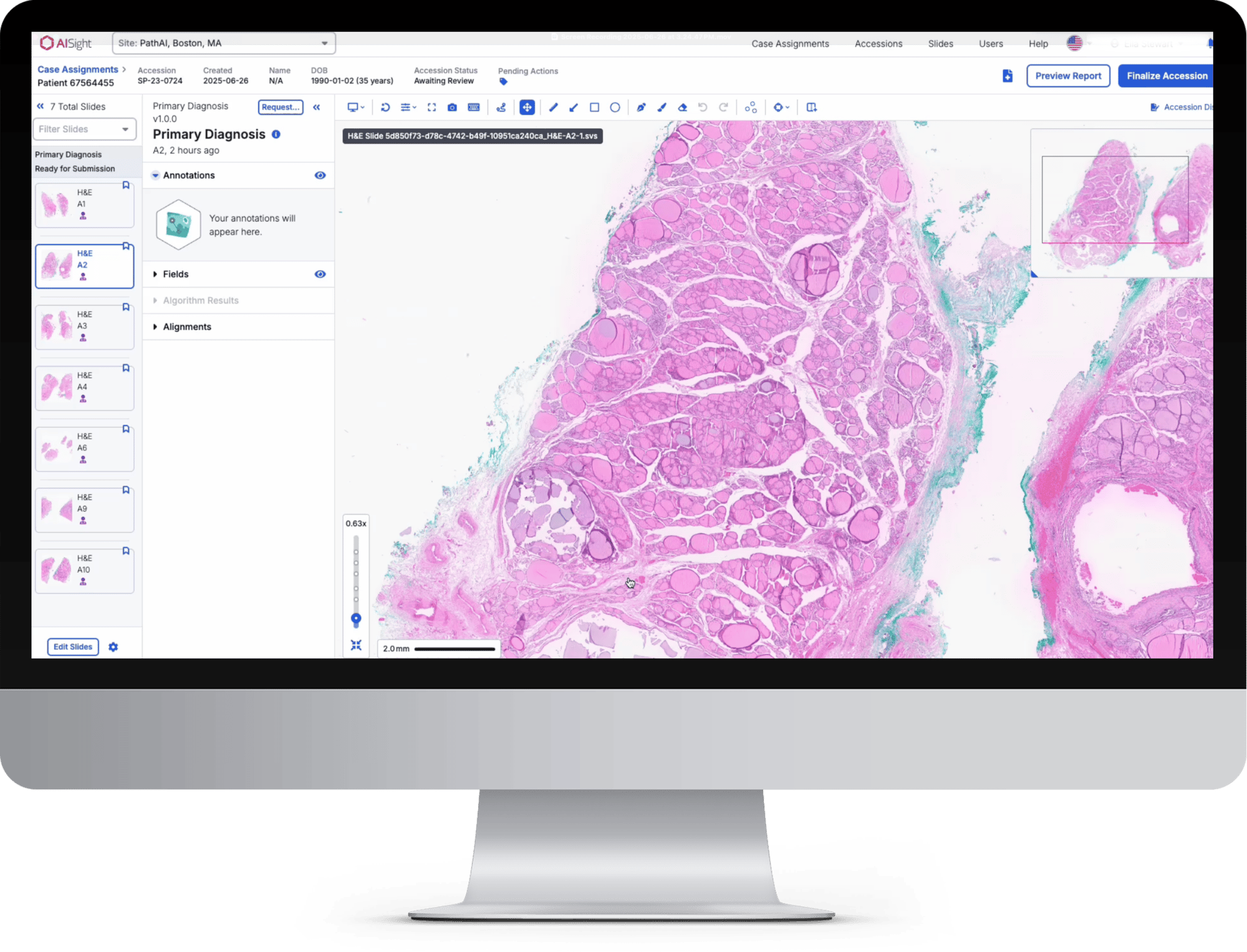
In this webinar, PathAI will show how AISight® Dx supports modern digital pathology workflows at scale, including seamless slide management, flexible collaboration, and interoperability across a broad range of whole-slide imaging scanners, and will explain what PCCP means for ongoing updates and customer value.
You’ll also see a live demonstration with case-based examples covering slide navigation, AISight Live for synchronous and asynchronous collaboration, annotation tools, and the caselist dashboard.
What you will learn
- Learn what it means for AISight® Dx to be the first digital pathology image management system cleared under an FDA 510(k) with an authorized Predetermined Change Control Plan (PCCP).
- Recognize the practical value of standardizing digital pathology workflows for pathologists and lab technicians, reducing variability, accelerating turnaround, and supporting quality and compliance.
- Experience a live demonstration featuring fast slide navigation and manipulation; measurements and annotations ; real-time and asynchronous collaboration with AISight Live; and caselist triage, search, filters, and workflow handoffs.
Speakers
MODERATOR & PRESENTER
Eric Walk, M.D.
Chief Medical Officer

PRESENTER
Carrene Plummer
Head of Regulatory and Quality

PRESENTER
Justin McCarthy
Head of Americas Commercial, DDx
Watch a brief overview of AISight Dx
Learn more about AISight® Dx here
PathAI Receives FDA Clearance for AISight® Dx Platform for Primary Diagnosis
PathAI Expands AISight® Dx Primary Diagnosis Clearance to Support VENTANA DP 200 and DP 600 Slide Scanners Through Predetermined Change Control Plan (PCCP)
PathAI and Moffitt Cancer Center Announce Strategic Collaboration to Deploy the AISight® Dx Digital Pathology Platform and Advance AI Diagnostics
CASE STUDY
AI-Assisted Titer Selection in Early Assay Development
- PathAI deployed IHC Explore on prostate cancer specimens stained with a novel, in-development assay
- IHC Explore quantifies staining intensity at single-cell resolution, enabling rapid assay characterization and titer optimization
- Continuous staining intensity measurement provides added value for next-generation biomarkers and precision medicine strategies
AISight® Dx is FDA-cleared for primary diagnosis in the US with the Hamamatsu NanoZoomer® S360MD, Leica Aperio® GT 450 DX and VENTANA® DP200 and DP600 slide scanners, and is CE‑IVD–marked for primary diagnosis in the EEA, UK, and Switzerland.
