Navigate the future of digital pathology with
AISight® Dx Digital
Pathology Platform
PathAI receives FDA
clearance for AISight® Dx
A complete, cloud‑native solution that centralizes case and image management on an intelligent platform.
PathAI has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for AISight Dx—PathAI’s digital pathology image management system—for use in primary diagnosis in clinical settings.
Introducing AISight® Dx
Interpret, share, and manage whole slide images of all sizes—from individual labs to large hospital networks
AISight Dx is a cloud-native digital pathology platform that streamlines diagnostic workflows and enables precision collaboration. With end-to-end automation, intelligent case routing, and robust image management tools, it supports accurate, efficient diagnoses across locations. Compliant with enterprise standards, AISight ensures PHI security and quality control for labs, hospitals, and academic centers.
AISight Dx: Overview
AISight Dx is a cloud-native digital pathology platform purpose-built to modernize anatomic pathology workflows. With intelligent case management, high-performance slide review, and seamless collaboration, AISight Dx helps labs boost efficiency, improve diagnostic consistency, and support high-quality patient care. FDA-cleared for use with Leica Aperio® GT 450 DX and Hamamatsu NanoZoomer® S360MD scanners, AISight Dx offers seamless interoperability, supporting organizations of any size across multiple sites.
Learn how our FDA‑cleared platform can help modern labs streamline workflows and foster precision collaboration.
Seamless Collaboration & Consults
AISight Dx brings seamless, real-time collaboration to digital pathology—enabling synchronized and asynchronous slide review, expert consults, and education in a secure, user-centric workspace. With a share-ready IMS and flexible access from anywhere, AISight Dx empowers the right specialists to connect and collaborate—when and where it matters most.
Seamless Collaboration & Consults
AISight Dx brings seamless, real-time collaboration to digital pathology—enabling synchronized and asynchronous slide review, expert consults, and education in a secure, user-centric workspace. With a share-ready IMS and flexible access from anywhere, AISight Dx empowers the right specialists to connect and collaborate—when and where it matters most.
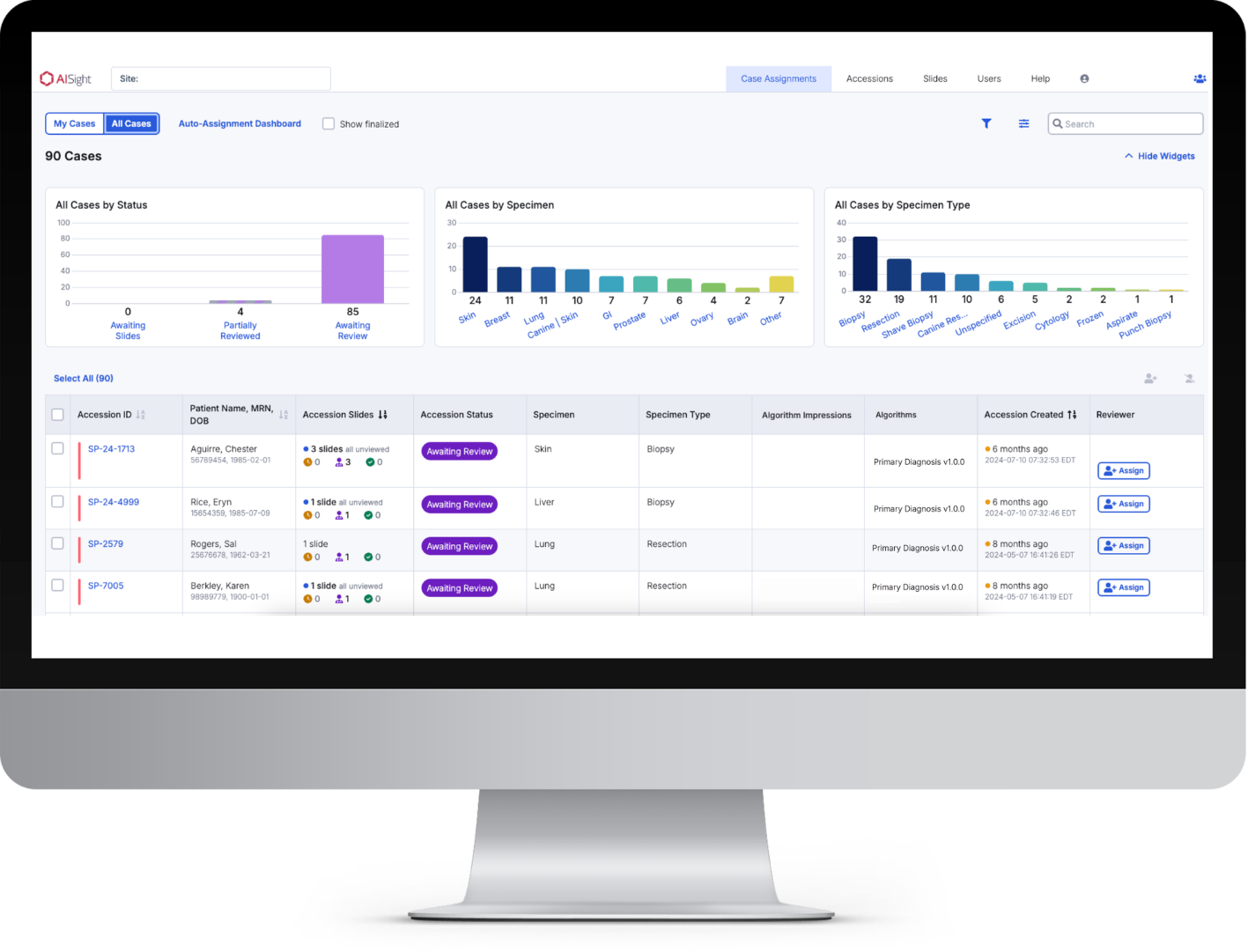
Intelligent Case Management
The Intelligent Caselist dashboard offers filterable workload charts, persistent search and filter settings across pages and sessions, and lets supervisors seamlessly assign or reassign cases to specific pathologist, allowing them to easily understand and navigate their daily worklist.
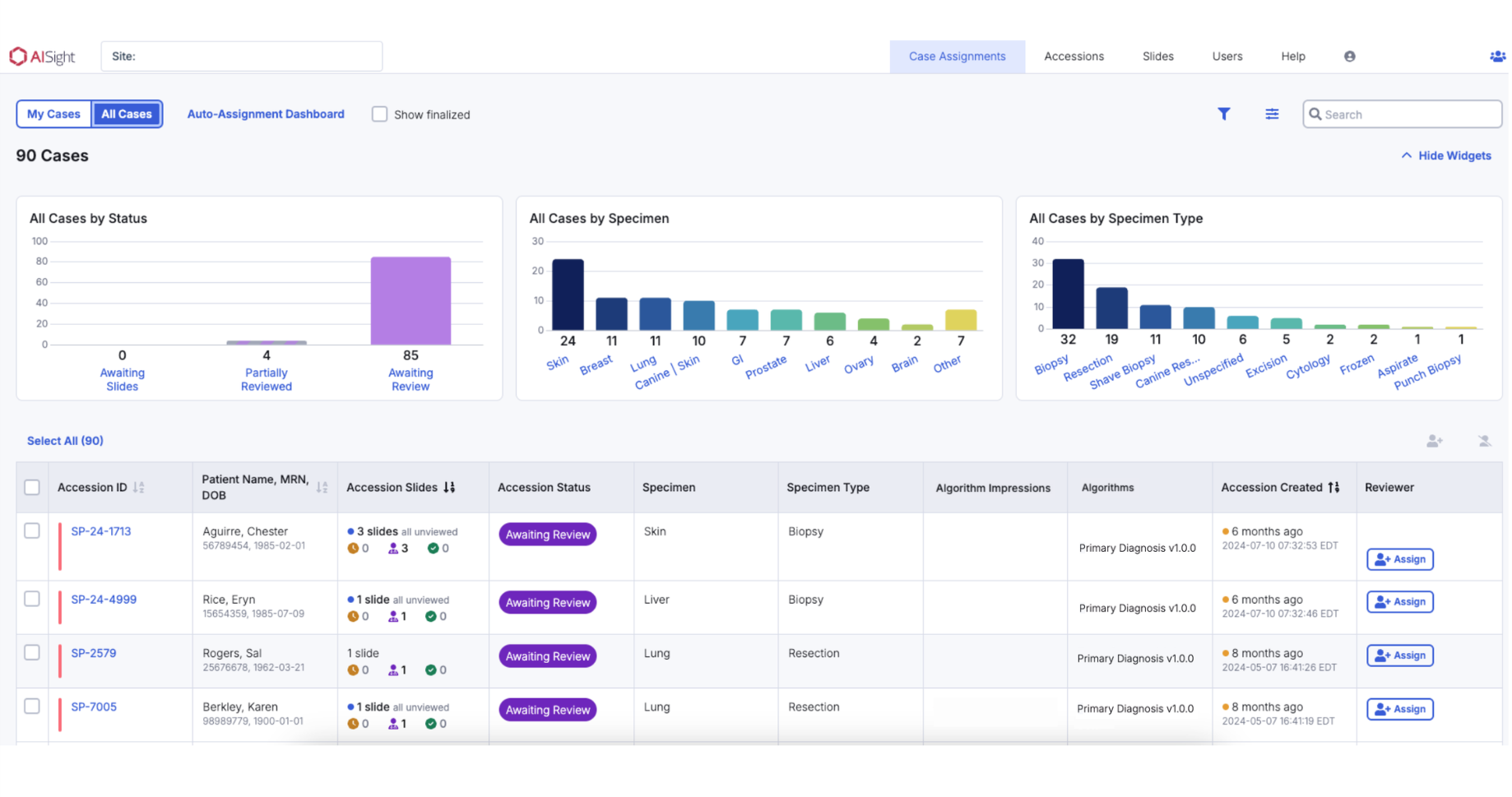
High-Performance Case Review
A high-performance slide viewer enables seamless review of whole slide images, offering a suite of tools about customizations to support an ideal workflow for each pathologist.
High-Performance Case Review
A high-performance slide viewer enables seamless review of whole slide images, offering a suite of tools about customizations to support an ideal workflow for each pathologist.
Enhanced Annotation Features
A robust set of tools including lines, arrows, shapes, and freehand drawing, combined with threaded comments and user tagging to support asynchronous slide review. Users can tag colleagues in specific comments, which triggers an immediate notification and brings them directly to the relevant annotation. This enables fast and focused collaboration across teams.
Schedule a free demo today
fuel your pathology workflows & research with AISight® Dx
AISight® Dx is FDA-cleared for primary diagnosis in the US with the Hamamatsu NanoZoomer® S360MD and Leica Aperio® GT 450 DX slide scanners,
and is CE‑IVD–marked for primary diagnosis in the EEA, UK, and Switzerland.

.png?width=2000&height=2000&name=Landing%20page%20header%20use%20graphic%20(1).png)