Drive the Evolution of AI & Digital Pathology
PathAI at USCAP 2026
USCAP 2026
Connect with us
Join us at USCAP 2026 as we highlight what’s next in AI-powered pathology. Stop by our booth for live demos, attend our scientific sessions, and connect with industry leaders at our exclusive event.
Be part of the momentum — where pathology meets the future.
PathAI Scientific Program
Posters
Abstract #: 2316
Abstract Title: AI-Powered Case Prioritization Expedites Review of Malignant Cases: A Controlled Crossover Study
Poster Board #: 187
Details: Poster Session on Tuesday, March 24, 2026 from 1:00 PM - 4:30 PM
Presenting Author: Benjamin Chen, MD
Abstract #: 2327
Abstract Title: AI-Powered Digital Scoring of Chromogenic In Situ Hybridization for Detection of MET Gene Amplification
Poster Board #: 181
Details: Poster Session on Tuesday, March 24, 2026 from 1:00 PM - 4:30 PM
Presenting Author: Ben Glass
Partner Presentation
Abstract #: 456
Abstract Title: AI-based Feature Extraction Reveals Distinct Histologic Signatures of Crohn’s-like Ileoanal Pouch Inflammation in Ulcerative Colitis
Poster Board #: 187
Details: Emerging Techniques and Artificial Intelligence on Tuesday, March 24, 2026 from 8:00 AM - 12:00 PM
Presentation Time: 8:00AM - 8:15AM
Presenting Author: Woong Kee Baek
Organization: Mount Sinai

Optimize Operational Efficiency
AISight automates workflows, prioritizes urgent cases, and streamlines tasks, reducing manual effort and enabling efficient case management with a modern, everyday-use interface.
Enhance Collaboration
AISight enables interdisciplinary communication and teamwork with both real-time consultation tools such as AISight Live and many different asynchronous collaboration tools.
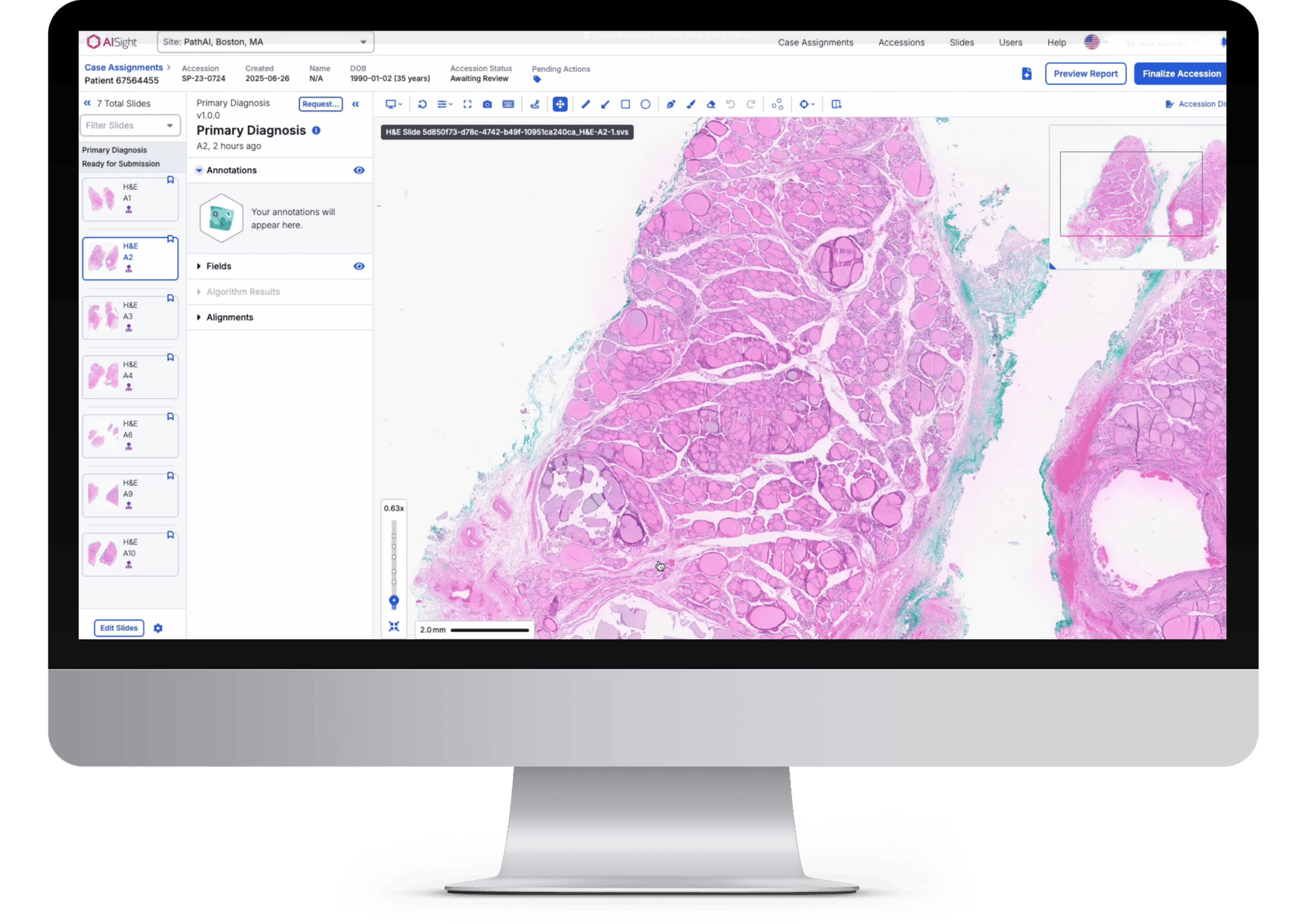
AI-Native IMS
AISight was intentionally designed to integrate AI in the slide viewer and beyond to enable next-generation efficiency through case prioritization, automated assignment, assisted reporting, and quality assurance.
Versatile Functionality
AISight supports a wide range of use cases including research, education, and training to streamline workflows and reduce the need for multiple systems.
Algorithms for Pathology Assessment
AISight further comes with the industry's largest menu of state-of-the-art and out-of-the-box algorithms across research, workflow optimization, assisted scoring, and biomarker quantification, and assistants use cases embedded directly into the IMS.

-1.png?width=1200&length=1200&name=DONE%20AISIght%20Brochure%20(3)-1.png)
-1.png?width=1200&length=1200&name=DONE%20AISIght%20Brochure%20(6)-1.png)
-1.png?width=1200&length=1200&name=DONE%20AISIght%20Brochure%20(5)-1.png)
-1.png?width=1200&length=1200&name=DONE%20AISIght%20Brochure%20(7)-1.png)
.png?width=1200&length=1200&name=DONE%20AISIght%20Brochure%20(8).png)
-1.png?width=1200&length=1200&name=DONE%20AISIght%20Brochure%20(1)-1.png)
-1.png?width=1200&length=1200&name=DONE%20AISIght%20Brochure%20(2)-1.png)
-1.png?width=1200&length=1200&name=DONE%20AISIght%20Brochure%20(4)-1.png)
CASE STUDY
AI-Assisted Titer Selection in Early Assay Development
- PathAI deployed IHC Explore on prostate cancer specimens stained with a novel, in-development assay
- IHC Explore quantifies staining intensity at single-cell resolution, enabling rapid assay characterization and titer optimization
- Continuous staining intensity measurement provides added value for next-generation biomarkers and precision medicine strategies
Explore how PathAI is transforming pathology through global partnerships, access the latest updates in our Newsroom.
PathAI's AIM-MASH AI Assist Becomes First AI-Powered Tool to Receive FDA Qualification for MASH Clinical Trials
PathAI and Moffitt Cancer Center Announce Strategic Collaboration to Deploy the AISight® Dx Digital Pathology Platform and Advance AI Diagnostics
PathAI Receives FDA Clearance for AISight® Dx Platform for Primary Diagnosis
Schedule a free demo at USCAP 2026 today
Fuel your pathology workflows & research with AISight®
Stay connected with PathAI, subscribe for the latest updates and marketing communications delivered straight to your inbox.
AISight® is for Research Use Only in the US. Not for use in diagnostic procedures. AISight® Dx is FDA-cleared for primary diagnosis in the US and CE-IVD marked for primary diagnosis in the EEA, UK, and Switzerland.
ArtifactDetect is a workflow tool available on AISight® and AISight® Dx. TumorDetect, AIM-Tumor Cellularity, AIM-MASH, AIM IHC Breast Panel, MET Predict, PathAssist Derm, AIM-HI UC, AIM-PD-L1 are for research use only. Not for use in diagnostic procedures.
