Introducing PathExplore™ Fibrosis
Unlock the fibrotic microenvironment directly from H&E whole slide images.
- Characterize the collagen & fiber composition of the tumor microenvironment to fuel oncology research for novel drug development
- Quantify fiber morphological features with high spatial specificity
Capture and analyze fibrosis, collagen, and fiber directly from routine pathology slides
Combined with PathExplore™ model outputs, this enables us to support customers exploring the tumor microenvironment beyond cellular structures, as well as those advancing drug development targeting the extracellular matrix.

Fast & Scalable FME Analysis:
Unlike traditional methods that require special stains and complex imaging technology, PathExplore™ Fibrosis leverages AI to quickly analyze routine H&E-stained slides, enabling large-scale studies and fast biomarker discovery.

Quantitative Insights:
The measurement of fibrosis, collagen, and fiber morphology directly from whole-slide images provides an unprecedented level of detail on tumor microenvironment features, offering new opportunities to explore tumor biology and therapeutic responses.

Integrate with Standard Pathology Workflows: By working with standard pathology workflows (H&E-stained slides), the algorithm democratizes advanced fibrosis analysis, allowing labs to integrate it easily without additional microscopy equipment or complex preparation techniques.
Fibers, collagen, and fibrosis are emerging biomarkers for oncology
The search for novel biomarkers for oncology and fibrosis has led to increased interest in studying fibers, particularly fibrosis and collagen fibers, because these structural elements of the tumor microenvironment (TME) play significant roles in cancer progression, metastasis, immune response, and drug resistance.
Here’s why fibers are gaining attention as valuable biomarkers:
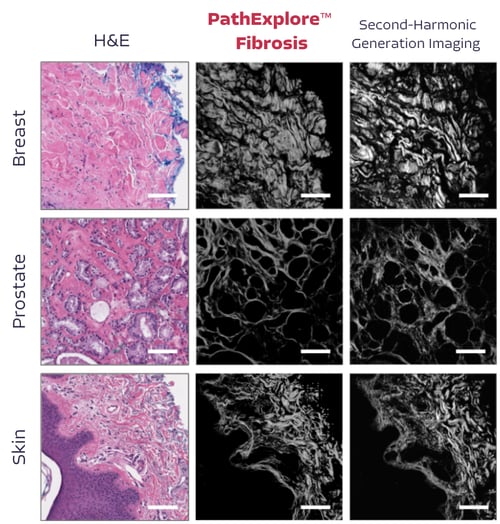
Most tumors develop abnormal fibrotic regions consisting of fibroblasts, immune cells, and a dense extracellular matrix (ECM). Fibers within the TME, such as collagen, contribute to the physical structure that surrounds and supports tumor cells. Increased fibrosis, or desmoplasia, can promote a rigid, dense environment, affecting how tumor cells grow, migrate, and invade surrounding tissues. Quantifying fibers allows researchers to better understand the extent of this structural change and its implications for tumor aggressiveness.
Collagen and other fibers influence immune cell infiltration into tumors. For example, high levels of collagen density and fiber alignment can create physical barriers that limit immune cell penetration, reducing the effectiveness of immune-based therapies. By studying fibers, researchers can gain insights into how fibrotic environments may inhibit or enhance immune response
Fiber composition and alignment can potentially affect how tumors respond to therapies. Dense, fibrotic tissues can act as barriers to drug delivery, making it challenging for therapeutic agents to reach tumor cells. Measuring fibrosis levels provides predictive information on how well a tumor might respond to certain treatments potentially allowing for more targeted treatment planning
The extracellular matrix (ECM) plays a crucial role in cancer progression, providing structural support and influencing how cells grow, migrate, and respond to therapies. Dense ECM regions, particularly those with high collagen content, often create barriers that limit drug delivery and shield tumors from immune cells. This makes the ECM an attractive target for novel cancer therapies, as disrupting these barriers can enhance the effectiveness of treatments and improve patient outcomes.
Studying fibrosis and collagen fibers in cancer research is challenging because traditional methods are:
- Labor-intensive
- Require specialized stains and equipment
- Difficult to scale
- Limits access for inclusion in TME studies
Multi-Dimensional TME Analysis
PathExplore™ solutions provide researchers with a comprehensive view of the TME. With the new PathExplore™ Fibrosis add-on, you can now ask and answer novel questions about the fibrotic microenvironment’s role in cancer progression and more.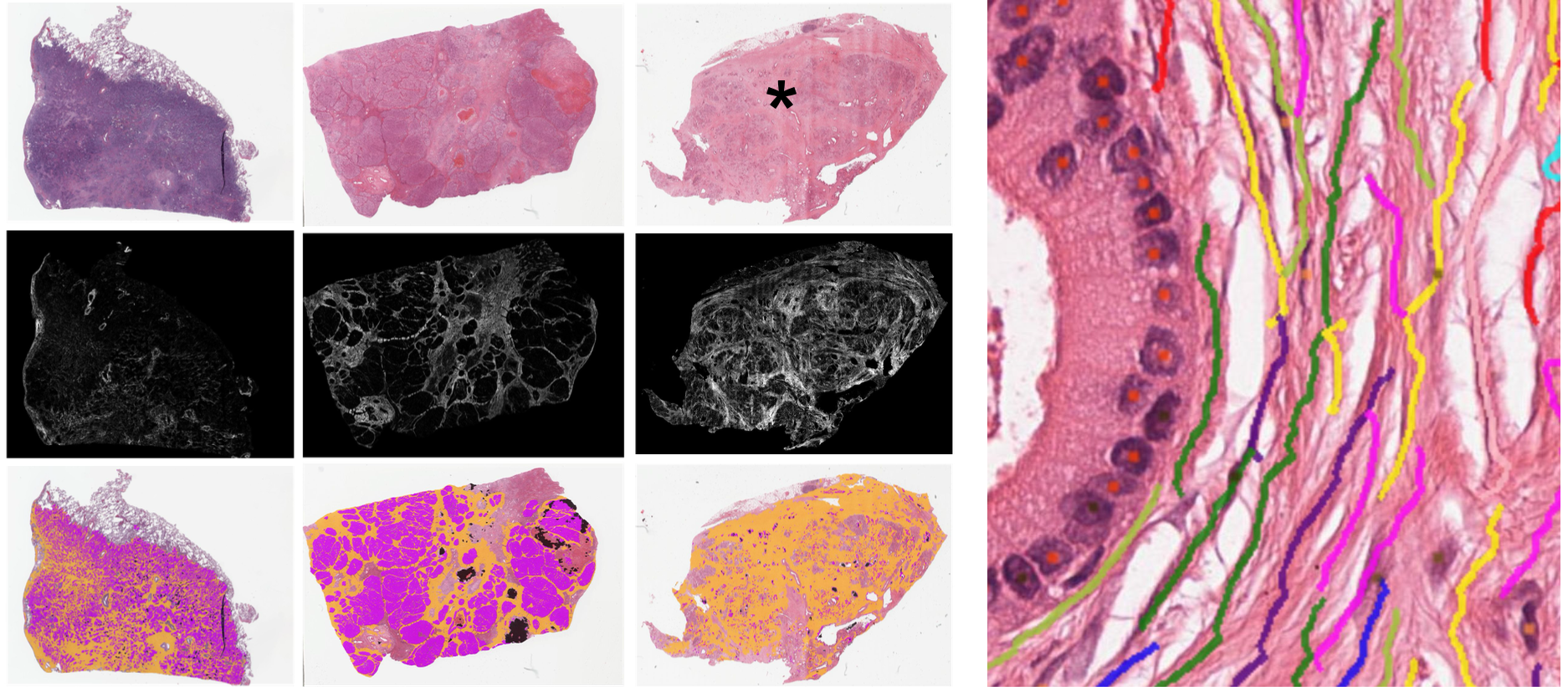
Effortlessly Scalable:
- Quantifies fibrosis, collagen, and fiber morphology from routine H&E-stained images, without the need for custom microscopy.
- Delivers insights into tumor biology and therapeutic responses, scaling analysis in oncology translational research.
- Enables new understanding of fibrosis and collagen as biomarkers and drug targets in cancer research.
- Enhances evaluation of drug efficacy and development of fiber-related therapies by examining fibrotic features of the tumor microenvironment (TME).
Access PathExplore™ Fibrosis today
-
- See for Yourself: try our self-serve demo for hands-on experience.
- Be First: Access PathExplore™ Fibrosis by reaching out at bd@pathai.com
Our content library
CASE STUDY
AI-Assisted Titer Selection in Early Assay Development
- PathAI deployed IHC Explore on prostate cancer specimens stained with a novel, in-development assay
- IHC Explore quantifies staining intensity at single-cell resolution, enabling rapid assay characterization and titer optimization
- Continuous staining intensity measurement provides added value for next-generation biomarkers and precision medicine strategies
Publication Collections
PathExplore™ and PathExplore™ Fibrosis are for research use only. Not for use in diagnostic procedures.