Advancing AI-Powered Pathology through strategic collaboration
Precision Pathology Network
-
Early Algorithm Access
-
Unlock New Revenue Pathway through Real World Data
-
Unlock New Evidence-Generation Opportunities
-
Accelerating Clinical Development with AI-Powered Pathology
-
Access to AI Precision-Medicine Tools & Frameworks
Precision Pathology Network
First-of-its-kind digital pathology network powered by PathAI’s AISight® platform expands access to latest AI products, unlocks real-world data value, and fuels collaborative research with biopharma across a broad lab network.
PathAI unveiled the Precision Pathology Network (PPN), the first network of digital anatomic pathology laboratories powered by its AISight® Image Management System (IMS). Built on a unified technology platform, the PPN provides early access to AI-powered pathology solutions developed with the biopharma industry, helps labs monetize real-world pathology data, supports biopharma-sponsored evidence-generation studies, and drives novel clinical development strategies leveraging AI-pathology.
Why Choose Precision Pathology Network?
Early Algorithm Access
Members will receive prioritized access to new algorithm products developed by PathAI. These next-generation tools support the future of precision biomarker testing and patient care, allowing PPN labs to offer them immediately upon commercial availability.
Unlock New Revenue Pathways Through Real World Data
Through a secure, standardized framework, member labs can contribute de-identified pathology data (whole slide images, reports, clinical/molecular data) for translational research and biopharma collaborations—unlocking fresh revenue streams.Unlock Revenue Opportunities in High-Quality Evidence Generation Studies
Achieving 'evidence generation readiness' is crucial, demanding consistent data collection and strict protocol adherence. While PathAI does not create readiness, we demystify the process, empowering labs to confidently compete for and win significant evidence generation business.Accelerating Clinical Development with AI-Powered Pathology
Ability to leverage PathAI’s proprietary algorithms, such as MET-Predict™, which identifies MET-amplified tumors with high accuracy, into PPN-member workflows to support patient identification and screening for clinical trial enrollment.Access to AI Precision-Medicine Tools & Frameworks
Members will benefit from the ability to access PathAI's market-leading biomarker discovery Explore™ product line via AISight, as well as the company’s PLUTO foundation model. Enabling novel biomarker research, grant collaboration, and even custom model co-development opportunities.Meet AISight®
A complete, cloud‑native solution that centralizes case and image management on an intelligent platform.
Streamline Workflows
Automates data organization, flags samples of interest, and simplifies batch processing, reducing manual steps and helping research teams handle large study datasets more efficiently
Enhance Collaboration
Enables interdisciplinary communication and teamwork with real-time consultation tools such as AISight Live, improving collaboration on complex cases
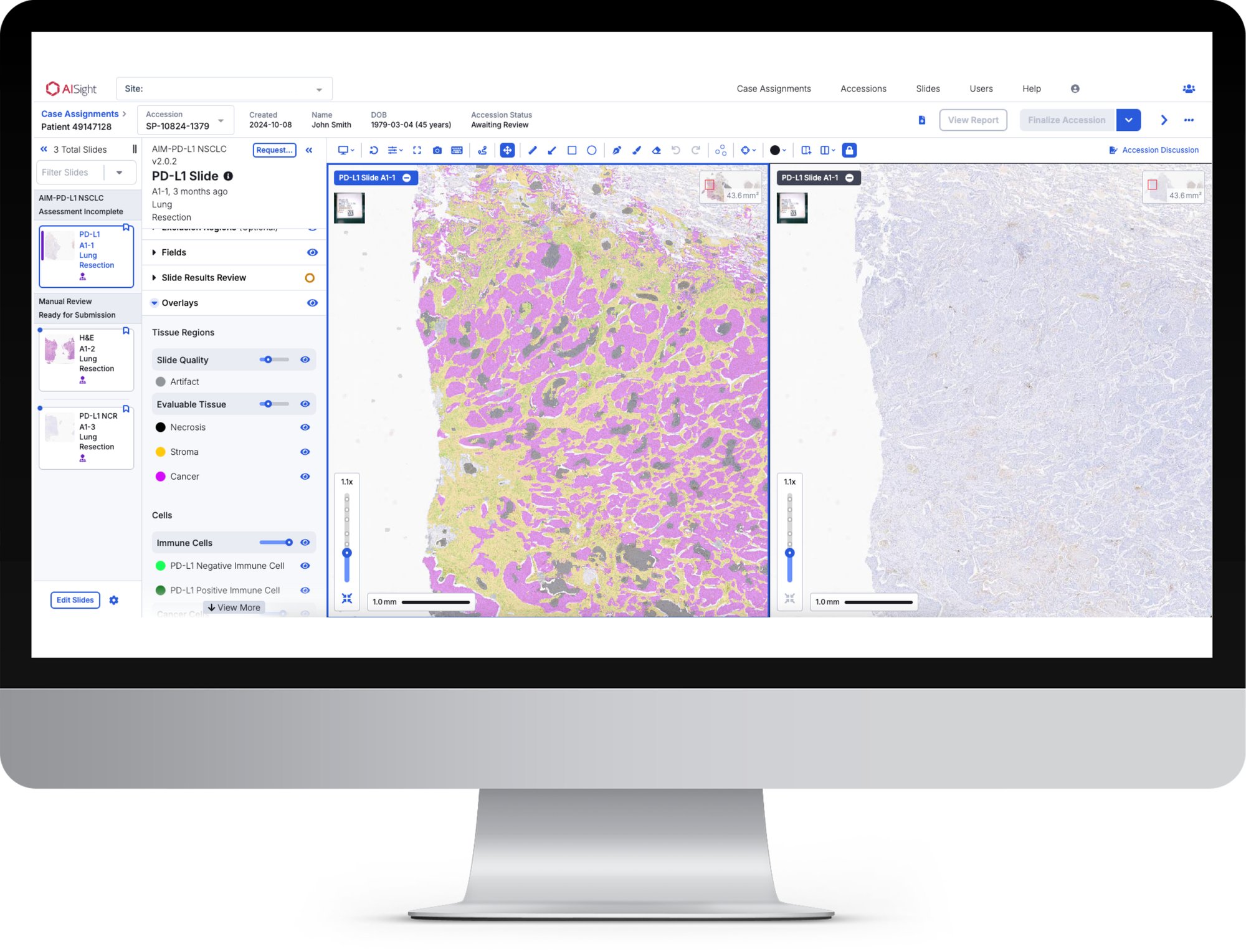
AI-Native IMS
Direct integration of AI algorithms enables next-generation workflow efficiency such as case prioritization, automated case assignment and artifact management
Versatile Functionality
Supports a wide range of use cases—including workflow optimization, histopathology research, education, and training—streamlining workflows and eliminating the need for multiple systems
CASE STUDY
AI-Assisted Titer Selection in Early Assay Development
- PathAI deployed IHC Explore on prostate cancer specimens stained with a novel, in-development assay
- IHC Explore quantifies staining intensity at single-cell resolution, enabling rapid assay characterization and titer optimization
- Continuous staining intensity measurement provides added value for next-generation biomarkers and precision medicine strategies
Collaborate
Work alongside top pathology labs and experts across the globe
Discover
Unlock new insights through real-world data and AI-powered tools
Advance
Accelerate innovation in diagnostics and clinical research
AISight® is for Research Use Only in the US; AISight® Dx is FDA-cleared for primary diagnosis in the US and is CE‑IVD–marked for primary diagnosis in the EEA, UK, and Switzerland. MET Predict™ and Explore™ Suite are for research use only and not for use in diagnostic procedures.
